‘Listening to light’ to transform diagnosis of skin cancer
Scientists are developing a new photonics device that listens to light and could be capable of detecting skin cancer and other diseases more accurately than ever before, eliminating the need for unnecessary and invasive biopsies.
With around 232,000 people around the world, estimated to have been diagnosed with malignant melanoma in 2012, and with 55,500 deaths, early diagnosis of the disease could see hundreds of thousands of lives saved over the next ten years, improve quality of life and reduce healthcare costs.
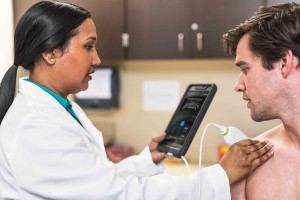
Traditionally, skin diseases are diagnosed visually by a physician using the naked eye or a magnifying glass and personal experience to make a decision. Invasive, uncomfortable, and potentially damaging procedures such as biopsies are often performed to confirm or exclude the presence of disease. This new breakthrough would give physicians an accurate and reliable way to objectively identify serious skin diseases for the first time.
“We are essentially listening to light, allowing us to see not just structures but molecules and biology on and under the skin, at depths and contrast never visualized before. It will enable physicians to make accurate and objective diagnosis of skin conditions for the first time.” said Professor Vasilis Ntziachristos, INNODERM Coordinator and Chair for Biological Imaging at the Technical University of Munich.
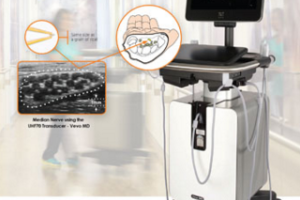
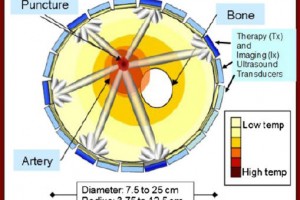
The method uses opto-acoustics, sending light waves of different wavelengths into the skin and detecting ultrasound waves generated within tissue in response to light absorption to build up an image of the skin tissue and specific molecules therein.
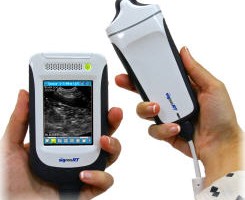
The prototype can visualize at depths up to 5mm under the skin, measures 4cm x 4cm x 7cm, no bigger than a small apple and can be placed on the skin to generate a high-resolution image in less than a minute. Being portable and of small form factor means that it could be used on expeditions or in remote areas of the world where a young doctor with little experience can make accurate, objective diagnoses.
“The device allows us to see blood vessels, skin oxygenation and potentially several novel pathophysiological features which are an integral area in the development of diseases. No one has ever been able to see like this before.” continued Professor Ntziachristos.
INNODERM, or Innovative Dermatology Healthcare based on Label-Free Spectral Optoacoustic Mesoscopy , combines the expertise of world-class engineers, scientists and clinicians in a consortium comprising 5 partners from 4 European countries.
The project has been awarded a grant of €3.8 million from Horizon 2020, the EU framework programme for research and innovation under the Photonics21 Public Private Partnership.
For more information: http://www.photonics21.org/index.php